Chinese Medical Journal Pulmonary and Critical Care Medicine Study Explores Idiopathic Pulmonary Fibrosis Pathogenesis
Understanding the cause of the lung scarring can lead to the development of biomarkers and therapeutics to identify and treat the disease
BEIJING, CHINA, August 28, 2024 /EINPresswire.com/ -- Pulmonary fibrosis is a condition which causes scarring of the lungs. As the disease progresses, lung tissue becomes thicker and stiffer limiting the exchange of gases. However, the cause of the scarring is not yet fully understood. However, researchers now shed light on the complex interplay between alveolar epithelial cells and fibroblasts in driving fibrosis progression. Understanding the mechanisms that contribute to the progression of fibrosis, holds promise for future treatments.Interstitial lung diseases (ILDs) refer to a range of conditions that damage the tissues between the air sacs (alveoli) and the capillaries in the lungs. While once considered rare, ILDs are now more common, particularly among those over the age of 50, affecting about 7% of people in this age group. One type of ILD is idiopathic pulmonary fibrosis (IPF), an irreversible and chronic condition where healthy lung tissue is gradually replaced with abnormal extracellular matrix (ECM) space or connective tissue (scarring of lung tissue). As the disease progresses, the tissue that surrounds the alveoli becomes thicker and stiffer, making it harder for the lungs to exchange oxygen and carbon dioxide during breathing. Studies show that, on average, people diagnosed with IPF only live for 2 to 3 years after diagnosis. The cause of the disease is not fully understood, but it is believed that abnormal wound-healing processes of the epithelial cells which line the alveoli, cause healthy cells to be replaced with scar tissue.
Given that the prevalence of IPF is increasing with an ageing population, a team of researchers led by Dr. Yihua Wang from the University of Southampton highlight the probable causes that contribute to fibrotic changes in the lung tissue. This paper was published in Volume 2, Issue 1 of the journal Chinese Medical Journal Pulmonary and Critical Care Medicine on March 2024.
“While an abnormal wound-healing response is strongly implicated in lung fibrosis initiation, factors that determine why fibrosis progresses rather than regular tissue repair occurs are not fully explained,” highlights Dr. Wang, the corresponding author of the study.
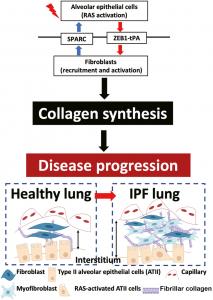
When a wound occurs, mesenchymal cells, particularly fibroblasts, proliferate at the site of the injury. These fibroblasts transform into myofibroblasts, which produce ECM components to heal the injury. However, in the case of IPF, there is excessive deposition of collagen and other ECM components, leading to scarring of the lung tissue. Individuals with pulmonary fibrosis have been found to have a higher number of fibroblasts compared to those with healthy lungs. It was previously thought that the excessive fibroblasts were produced due to micro-injuries to the alveolar epithelial cells (AECs) caused by various risk factors such as infection, environmental or occupational conditions, smoking, and genetic mutations. However, recent evidence suggests that dysfunction and loss of integrity of the AECs can contribute to the abnormal deposition of collagen and ECM components.
In this review, the researchers underscore the complex interplay between epithelial cells and fibroblasts in driving fibrosis progression. They point to the development of a pro-fibrotic microenvironment which creates a self-sustaining fibrosis cycle. Damaged epithelial cells secrete various growth factors such as transforming growth factor- 𝛽 (TGF- 𝛽 ), tumor necrosis factor- 𝛼 (TNF- 𝛼 ), osteopontin, angiotensinogen, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), endothelin-1, and insulin-like growth factor 1 (IGF-1), which enhance the accumulation of myofibroblasts. In this pro-fibrotic microenvironment, the myofibroblasts through various mediators such as angiotensin II (ANG II) or reactive oxygen species (ROS), induce apoptosis (cell death) in epithelial cells. This creates a cycle where tissues need to be constantly repaired, leading to the progression of fibrosis.
Injured AECs, can also undergo an epithelial-to-mesenchymal transition (EMT) where they can take on properties of the myofibroblasts, further contributing to fibrosis. In IPF, RAS (Renin-Angiotensin System) activation plays a significant role in driving fibroblast activation. AECs activated by RAS, release signaling molecules called paracrine factors that act on neighboring fibroblasts in the surrounding tissue.
Crucially the researchers propose that the dysfunction in the paracrine signaling loop between Type II alveolar epithelial cells (ATII cells) and fibroblasts contributes to fibrosis progression in the lung. Paracrine regulators like secreted protein acidic and rich in cysteine (SPARC) released from activated fibroblasts can damage the alveolar epithelial barrier and cause a chronic wound-healing response.
“We have provided strong evidence demonstrating that in both TGF𝛽- activated normal lung fibroblasts and IPF fibroblasts, paracrine SPARC signaling not only dysregulates the alveolar epithelial barrier integrity, but also activates EGFR/RAS/ERK signaling in ATII cells to maintain a chronic wound-healing phenotype,” says Associate Prof. Wang.
By shedding light on the progression of IPF, the finding can help develop therapeutics to slow or stop the disease and biomarkers to identify it. “Dysregulated bi-directionally controlled paracrine signaling between epithelial and mesenchymal cells is a core feature of a pro-fibrotic microenvironment. EMT-related targets on epithelial cells and fibroblasts have therapeutic promise in fibrotic diseases,” says Dr. Wang.
***
Reference
Title of original paper: Dysregulated bidirectional epithelial–mesenchymal crosstalk: A core
determinant of lung fibrosis progression
Journal: Chinese Medical Journal Pulmonary and Critical Care Medicine
DOI: 10.1016/j.pccm.2024.02.001
Peifang Wei
Chinese Medical Journal Pulmonary and Critical Care Medicine
+86 138 1092 3769
Weipeifang@cma.org.cn
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release