
Blood Cancer Treatment Market Sees Accelerated Growth Across the 7MM Amid Rising Incidence and Breakthroughs | DelveInsight
The blood cancer market is experiencing robust growth, driven by rising incidence rates and advancements in targeted therapies and immuno-oncology. Increasing adoption of CAR-T cell therapies, monoclonal antibodies, and personalized medicine is transforming treatment outcomes.
/EIN News/ -- New York, USA, May 12, 2025 (GLOBE NEWSWIRE) -- Blood Cancer Treatment Market Sees Accelerated Growth Across the 7MM Amid Rising Incidence and Breakthroughs | DelveInsight
The blood cancer market is experiencing robust growth, driven by rising incidence rates and advancements in targeted therapies and immuno-oncology. Increasing adoption of CAR-T cell therapies, monoclonal antibodies, and personalized medicine is transforming treatment outcomes.
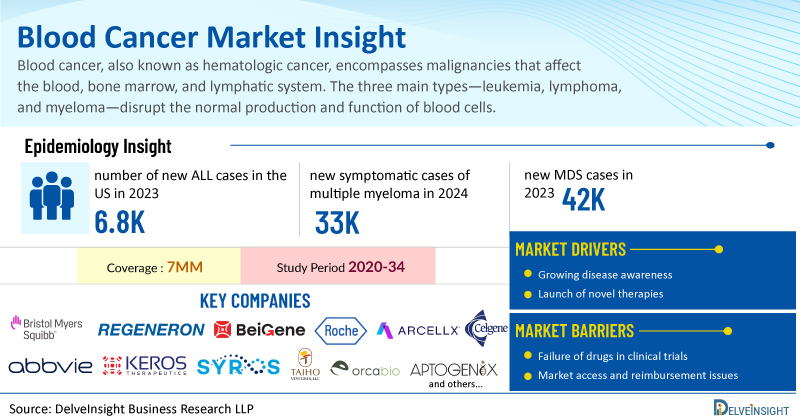
Blood cancer, also known as hematologic cancer, encompasses malignancies that affect the blood, bone marrow, and lymphatic system. The three main types—leukemia, lymphoma, and myeloma—disrupt the normal production and function of blood cells, often leading to anemia, immunodeficiency, and clotting issues. These cancers can be aggressive or slow-growing, with causes ranging from genetic mutations to environmental exposures. Technological advances have improved diagnosis and treatment, yet many patients still face relapses or resistance to therapy, making blood cancer a complex and evolving medical challenge.
Globally, blood cancers represent a significant portion of the cancer burden. Leukemia alone accounts for over 474,000 new cases and 311,000 deaths annually, while lymphomas are among the most common cancers in children and young adults. The patient burden is considerable, not only due to the life-threatening nature of the disease but also because of the prolonged treatments, frequent hospitalizations, and the emotional toll on patients and caregivers. High treatment costs, limited access to advanced therapies in low-resource settings, and long-term side effects contribute to both physical and financial strain, underscoring the need for more accessible, durable, and less toxic treatment options.
DelveInsight has expertise in the oncology market, and an experienced team handles the blood cancers domain proficiently. DelveInsight has recently released a series of epidemiology-based market reports on different types of blood cancers, including Leukemia, Lymphoma, Multiple Myeloma, Myelodysplastic Syndromes (MDS), and Myeloproliferative Neoplasms (MPN). These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s dive deeply into the market assessment of these blood cancer types individually.
Leukemia Market
Leukemia is a type of cancer that originates in the bone marrow and affects the blood and lymphatic systems. It results from the uncontrolled proliferation of abnormal white blood cells, which can crowd out healthy blood cells and impair immune function. There are four main types of leukemia: acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and chronic myeloid leukemia (CML), each differing by the speed of progression and the type of blood cells affected.
Acute Lymphocytic Leukemia Market
Acute lymphocytic leukemia (ALL), also known as acute lymphoblastic leukemia, is a blood and bone marrow cancer that arises from immature lymphocytes. It is characterized by the overproduction of abnormal lymphoblasts or leukemic blasts. This excessive cell growth disrupts normal blood cell production, leading to symptoms such as anemia, frequent infections, and increased bruising or bleeding due to the deficiency of healthy red blood cells, white blood cells, and platelets.
Among the seven major markets, the United States reported the highest number of new ALL cases, with 6,800 incident cases in 2023, a figure expected to rise over the forecast period. In the US, B-cell ALL represented about 85% of cases, while T-cell ALL made up roughly 15%.
The treatment landscape for ALL has evolved significantly with the introduction of targeted therapies such as tyrosine kinase inhibitors against BCR-ABL1 (e.g., GLEEVEC [imatinib mesylate], SPRYCEL [dasatinib], and ICLUSIG [ponatinib]), CD20-targeting monoclonal antibodies (RITUXAN), CD22-directed antibody-drug conjugates (BESPONSA), bispecific antibodies (BLINCYTO), and CD19-directed CAR T-cell therapies (KYMRIAH and TECARTUS), among others.
According to DelveInsight, the ALL market across the 7MM was valued at USD 1.6 billion in 2023, with the US accounting for around USD 1.2 billion of that total. The market is expected to grow further by 2034, with BLINCYTO currently leading the therapeutic segment, highlighting the significant commercial potential of ALL therapies.
The acute lymphocytic leukemia market is driven by a combination of factors, including advancements in treatment options, increasing incidence rates, and the rising demand for targeted therapies. As the understanding of the disease's molecular and genetic underpinnings improves, the development of novel therapies, such as monoclonal antibodies, CAR-T cell therapies, and small-molecule inhibitors, is gaining traction. These therapies are designed to offer more personalized and effective treatment options, addressing the limitations of traditional chemotherapy. Additionally, the expanding adoption of minimal residual disease monitoring techniques is improving early detection and treatment outcomes, fostering market growth.
Discover more about the ALL market in detail @ Acute Lymphocytic Leukemia Market Report
Acute Myeloid Leukemia Market
Acute myeloid leukemia (AML), also referred to as acute myelocytic leukemia, acute myelogenous leukemia, or acute granulocytic leukemia, is a condition marked by the rapid and uncontrolled growth of immature myeloid cells in the bone marrow, bloodstream, and occasionally in other organs. In 2024, the highest incidence of AML in the United States was reported among individuals aged 75 and older, comprising nearly 30% of cases, followed by those aged 65–74.
The most frequent genetic mutation seen in AML is in the NPM1 gene, present in roughly 40% of U.S. cases. Initial treatment during remission induction typically includes combination chemotherapy with cytarabine. For patients with FLT3 mutations, targeted therapies such as RYDAPT (midostaurin) or VANFLYTA (quizartinib) may be added, and MYLOTARG (gemtuzumab ozogamicin) is another option. In cases where the central nervous system (CNS) is affected, intrathecal chemotherapy using cytarabine or methotrexate is administered.
Older patients or those unfit for intensive chemotherapy may receive targeted agents like enasidenib, gilteritinib, or venetoclax, often combined with low-dose chemotherapeutics such as azacitidine or decitabine. Supportive care remains a critical component throughout treatment to manage side effects and CNS involvement.
Notable companies developing AML therapies include AbbVie and SELLAS Life Sciences. Promising candidates in development include AbbVie’s Pivekimab Sunirine and ABBV-787, as well as SELLAS Life Sciences’ Galinpepimut-S.
For a comprehensive view of the AML market, check out the Acute Myeloid Leukemia Market Assessment
Chronic Lymphocytic Leukemia Market
Chronic lymphocytic leukemia (CLL) can be broadly categorized into two types, depending on whether it originates from B cells or T cells. Over 95% of CLL cases are of the B-cell variety, while T-cell CLL is much rarer, accounting for only 1–5% of cases.
Treatment options for CLL span immunotherapy, chemotherapy, targeted therapy, radiation, surgery, and leukapheresis. Key drug classes used in its management include Bruton tyrosine kinase (BTK) inhibitors like IMBRUVICA and CALQUENCE; BCL-2 inhibitors such as VENCLEXTA/VENCLYXTO; monoclonal antibodies including RITUXAN, ARZERRA, and GAZYVA; PI3K inhibitors like ZYDELIG; and the recently approved CAR T-cell therapy BREYANZI. Additionally, several promising candidates in clinical development are showing strong preliminary results and continue to be evaluated in trials.
Pharmaceutical companies are actively pursuing innovative treatments to address the unmet needs in CLL care. Notable players in this space include Merck (nemtabrutinib), Ascentage Pharma (lisaftoclax), BeiGene (sonrotoclax), AbbVie/Genmab (epcoritamab), Johnson & Johnson Innovative Medicine (JNJ-64264681/JNJ-4681), and Starton Therapeutics (STAR-LLD), among others.
In summary, the CLL market is expected to undergo considerable transformation between 2025 and 2034 with the introduction of new therapies. However, growth may be constrained by challenges such as limited access to diagnostics, the complexity of treatment options, high costs of advanced cell therapies, disease progression, and the impact on patients’ quality of life.
Discover more about CLL drugs in development @ Chronic Lymphocytic Leukemia Clinical Trials
Chronic Myeloid Leukemia Market
Chronic myeloid leukemia (CML) is a type of blood cancer that originates in the bone marrow and is characterized by the overproduction of abnormal white blood cells. It is primarily associated with the Philadelphia chromosome, a genetic abnormality resulting in the BCR-ABL fusion gene, which leads to unchecked cell division. CML typically progresses through three phases, chronic, accelerated, and blast crisis, with most patients diagnosed during the chronic phase. Globally, CML accounts for approximately 15% of all adult leukemias, with an incidence rate of about 1–2 cases per 100,000 individuals annually. While more common in older adults, it can occur at any age and has a slight male predominance.
The treatment landscape for CML has dramatically transformed over the past two decades, primarily due to the advent of tyrosine kinase inhibitors such as imatinib, dasatinib, and nilotinib, which target the BCR-ABL fusion protein. These therapies have turned CML into a manageable chronic condition for many patients, with significantly improved survival rates and quality of life.
The CML therapeutics market is expected to continue its expansion, driven by increasing awareness, better diagnostics, and rising healthcare expenditure. However, market dynamics are evolving with the entry of generics, patent expirations of first-generation TKIs, and the development of next-generation therapies that address resistance or intolerance to existing drugs. Additionally, increasing focus on treatment-free remission and personalized medicine is shaping future growth and innovation in the CML space.
To gain a deeper understanding of the CML market, be sure to explore the Chronic Myeloid Leukemia Market Outlook
Lymphoma Market
Lymphoma is a type of cancer that begins in the lymphatic system, which is part of the body’s immune system responsible for fighting infections and maintaining fluid balance. It arises from lymphocytes, a type of white blood cell, and is broadly categorized into two main types: Hodgkin lymphoma and non-Hodgkin lymphoma. Each type behaves differently and requires distinct treatment approaches.
Hodgkin’s Lymphoma Market
Hodgkin lymphoma, also known as Hodgkin disease, is a form of cancer that begins in the lymphatic system and is marked by the presence of distinctive Reed-Sternberg cells. It belongs to one of the two main categories of lymphoma, the other being non-Hodgkin lymphoma, and is recognized for its high potential for cure, especially when detected and treated early.
There are two primary subtypes of Hodgkin lymphoma: classic Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma. Although it is uncommon in children under five, it is the most frequently diagnosed cancer in teenagers aged 15 to 19.
Treatment strategies typically combine chemotherapy, radiation therapy, and targeted therapies, customized according to the stage and type of the disease. For advanced cases, chemotherapy protocols such as ABVD (which includes Adriamycin, Bleomycin, Vinblastine, and Dacarbazine) are widely used. Targeted agents like ADCETRIS (brentuximab vedotin) and immune checkpoint inhibitors such as OPDIVO (nivolumab) and KEYTRUDA (pembrolizumab) are crucial, especially in treating patients whose disease has relapsed or is resistant to standard treatments.
Among the leading companies in Hodgkin lymphoma drug development is ADC Therapeutics, which is advancing camidanlumab tesirine, a novel ADC that targets CD25, a protein often found on classical Hodgkin lymphoma cells. This therapy is being closely watched for its potential to benefit patients with relapsed or refractory cHL, especially those unresponsive to existing treatments like brentuximab vedotin and PD-1 inhibitors. It is currently undergoing Phase II clinical trials.
Explore in-depth for a comprehensive understanding of the Hodgkin’s Lymphoma Clinical Trials
Non-Hodgkin’s Lymphoma Market
Non-Hodgkin’s Lymphoma (NHL) is a diverse group of blood cancers that originate in the lymphatic system, primarily affecting lymphocytes, a type of white blood cell. Unlike Hodgkin’s lymphoma, NHL is characterized by the absence of Reed-Sternberg cells. The disease manifests in multiple subtypes, ranging from indolent forms like follicular lymphoma to aggressive variants such as diffuse large B-cell lymphoma (DLBCL), the latter being the most common subtype. Globally, NHL accounts for approximately 4% of all cancers, with over 500,000 new cases diagnosed annually. Incidence rates vary across regions, with higher rates observed in developed countries, partly due to better diagnostic capabilities and longer life expectancy.
The treatment landscape for NHL has evolved significantly, with standard care traditionally including chemotherapy (CHOP regimen), immunotherapy (notably rituximab), radiation, and hematopoietic stem cell transplantation. In recent years, the advent of targeted therapies and immuno-oncology has transformed outcomes, particularly with the rise of CAR T-cell therapies (e.g., YESCARTA and KYMRIAH) and antibody-drug conjugates (e.g., POLIVY).
Major pharmaceutical players such as AbbVie, Genmab, Merck, Roche, Xencor, Janssen, Denovo Biopharma, Calithera Biosciences, IMV, Biogen, Autolus Therapeutics, Allogene Therapeutics, Novartis, Miltenyi Biomedicine, Regeneron Pharmaceuticals, Debiopharm, Seagen, Takeda, AstraZeneca, and Gilead Sciences, among others, are at the forefront of NHL therapeutic development. The NHL market is poised for robust growth, driven by increasing disease prevalence, aging populations, and high demand for innovative therapies. However, challenges such as high treatment costs, relapse rates, and heterogeneity in treatment responses continue to shape market dynamics and foster ongoing R&D investments.
Explore the Non-Hodgkin Lymphoma Market Outlook for in-depth market insights
Multiple Myeloma Market
Multiple myeloma is a cancer marked by the unchecked growth of clonal plasma cells, leading to a range of complications, organ damage, and ultimately death. In 2024, over 33,000 new symptomatic cases were reported in the US, with this number projected to grow at a moderate CAGR from 2025 to 2034.
The treatment landscape for multiple myeloma is rapidly evolving, particularly with the increasing use of monoclonal antibodies in newly diagnosed patients. DARZALEX maintains a strong market position relative to its competitors. Many pipeline therapies are likely to be used in combination with DARZALEX rather than directly competing with it. Johnson & Johnson is investigating sequencing DARZALEX with TECVAYLI, CARVYKTI, and TALVEY.
SARCLISA, a recently approved CD38 antibody, has seen quick uptake, although DARZALEX benefits from a substantial head start. Both drugs are being used in quadruplet regimens for patients eligible and ineligible for transplants, with competition intensifying, especially in the non-transplant-eligible segment, where most clinical data still comes from transplant-eligible groups. Although both DARZALEX and EMPLICITI (from Bristol Myers Squibb and AbbVie) entered the market in the same month, DARZALEX has clearly pulled ahead in terms of commercial success.
Several promising therapies are in development, including Mezigdomide (Bristol Myers Squibb/Celgene), Linvoseltamab and REGN7945 (Regeneron), BGB-11417 (BeiGene), Cevostamab (Roche), and CART-ddBCMA (Arcellx), among others. In 2024, the US market size for first-line, transplant-ineligible multiple myeloma therapies was around USD 3.4 billion, with Daratumumab expected to generate the highest revenue by 2034.
The multiple myeloma market growth is anticipated to surge in the coming years, driven by rising incidence rates, earlier use and broader labels for current therapies, strong uptake of next-generation treatments like CAR-T cells and bispecific antibodies, a robust pipeline, and increasing R&D investment.
Discover which therapies are expected to grab the major multiple myeloma market share @ Multiple Myeloma Market Report
Myelodysplastic Syndromes (MDS) Market
Myelodysplastic syndrome (MDS) represents a diverse group of blood cancers, traditionally characterized as a clonal disorder of hematopoietic stem cells that results in abnormal cell development (dysplasia) and inefficient blood cell production in the bone marrow. In MDS, bone marrow cells fail to mature into functional blood cells, instead remaining in an underdeveloped state within the marrow.
According to DelveInsight, approximately 42,000 new MDS cases were reported across the 7MM in 2023, with this figure projected to rise by 2034. In the United States, the RAEB/MDS-EB subtype was the most prevalent, with nearly 7,000 cases recorded in 2023, and this number is also expected to grow over time.
Current standard treatments for MDS include supportive care, pharmacologic therapy, and stem cell transplantation. Patients suffering from symptoms due to low blood cell counts are often managed with supportive treatments to alleviate symptoms and enhance quality of life. Drug therapies may help slow disease progression, while aggressive treatment with chemotherapy followed by allogeneic stem cell transplantation can potentially lead to a cure in select patients.
Recent FDA-approved therapies for MDS include RYTELO (imetelstat), TIBSOVO (ivosidenib), REBLOZYL (luspatercept-aamt), INQOVI (decitabine and cedazuridine), GLEEVEC (imatinib), and VIDAZA (azacitidine), among others.
The MDS treatment pipeline is highly active, with several promising therapies in development. These include VENCLEXTA (AbbVie), bexmarilimab (Faron Pharmaceuticals), KER-050 (Keros Therapeutics), tamibarotene (Syros Pharmaceuticals), ASTX030 (a combination of cedazuridine and azacitidine by Taiho Oncology), Orca-T (Orca Bio), JNJ-64619178, JNJ-74856665 (Johnson & Johnson Innovative Medicine), and asunercept (Apogenix), among others.
In summary, the MDS treatment landscape is poised for substantial growth from USD 2.8 billion in 2023 in the 7MM by 2034, driven by the introduction of novel therapies currently under clinical evaluation. However, challenges such as limited treatment suitability for elderly patients and reliance on blood transfusions may hinder market growth.
Download the report to understand which factors are driving the myelodysplastic syndrome market trends @ Myelodysplastic Syndrome Market Insights
Myeloproliferative Neoplasms (MPN) Market
Myeloproliferative Neoplasms (MPNs) are a group of blood cancers characterized by the excessive production of blood cells in the bone marrow. This category includes conditions like chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and primary myelofibrosis (PMF).
The pathogenesis of MPNs is primarily driven by mutations in hematopoietic stem cells, with the JAK2 V617F mutation being the most common genetic alteration found in many of these disorders. The global incidence of MPNs varies but is generally low, with an estimated annual incidence of 1-2 cases per 100,000 people for most MPNs, though it increases with age. CML, PV, and ET have relatively higher incidences compared to PMF, which is rarer.
Treatment options for MPNs have evolved significantly over the years. For diseases like CML, targeted therapies like TKIs, such as imatinib, have revolutionized treatment, offering patients long-term survival. In PV and ET, treatment focuses on managing symptoms and preventing thrombotic events, with options including phlebotomy, aspirin, and the use of cytoreductive agents like hydroxyurea. For PMF, therapies like ruxolitinib, a JAK1/2 inhibitor, have been shown to alleviate symptoms and improve quality of life, though the treatment landscape remains challenging. Stem cell transplantation remains an option for high-risk patients, but it is associated with significant risks.
The market dynamics of MPNs are influenced by a combination of factors, including the rarity of the diseases, the high cost of targeted therapies, and the ongoing development of novel treatments. The market for MPN therapies is growing, driven by increasing awareness, better diagnosis, and the emergence of new drugs.
However, due to the complexity and heterogeneity of MPNs, the treatment landscape remains fragmented. While JAK inhibitors and other targeted therapies are gaining traction, there remains a significant unmet need for therapies that can address the full spectrum of disease progression and improve long-term outcomes. The ongoing clinical trials and research into combination therapies are expected to further expand treatment options and shape the future market for MPN treatments.
To access a complete analysis of the MPN market, visit Myeloproliferative Neoplasms Market Assessment
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

